Chemical Recycling
Chemical Recycling
Plastics are mostly petrochemicals, made from natural gas or petroleum, a type of oil. Chemical engineers refine the petroleum which goes through a heating process to develop ethylene and propylene, which are the chemical building blocks for many plastics. These chemicals are then combined with other chemicals to produce a polymer.
Chemical recycling is any process that turns plastic polymers back into individual monomers (a molecule that can be bonded to other identical molecules to form a polymer). During the chemical recycling process, the chemical building blocks that make up the plastic are recovered. Once the plastics have been returned to these fundamental building blocks, it can, in some cases, be re-polymerized endlessly, giving them the qualities of brand-new, or virgin, resin.
Chemical recycling is best suited for hard to recycle, multilayered or heavily contaminated plastics. The main benefit of a chemical recycling process is that it is more tolerant of contamination, and it yields polymers that are identical to the original, eliminating downcycling.
Types of Chemical Recycling
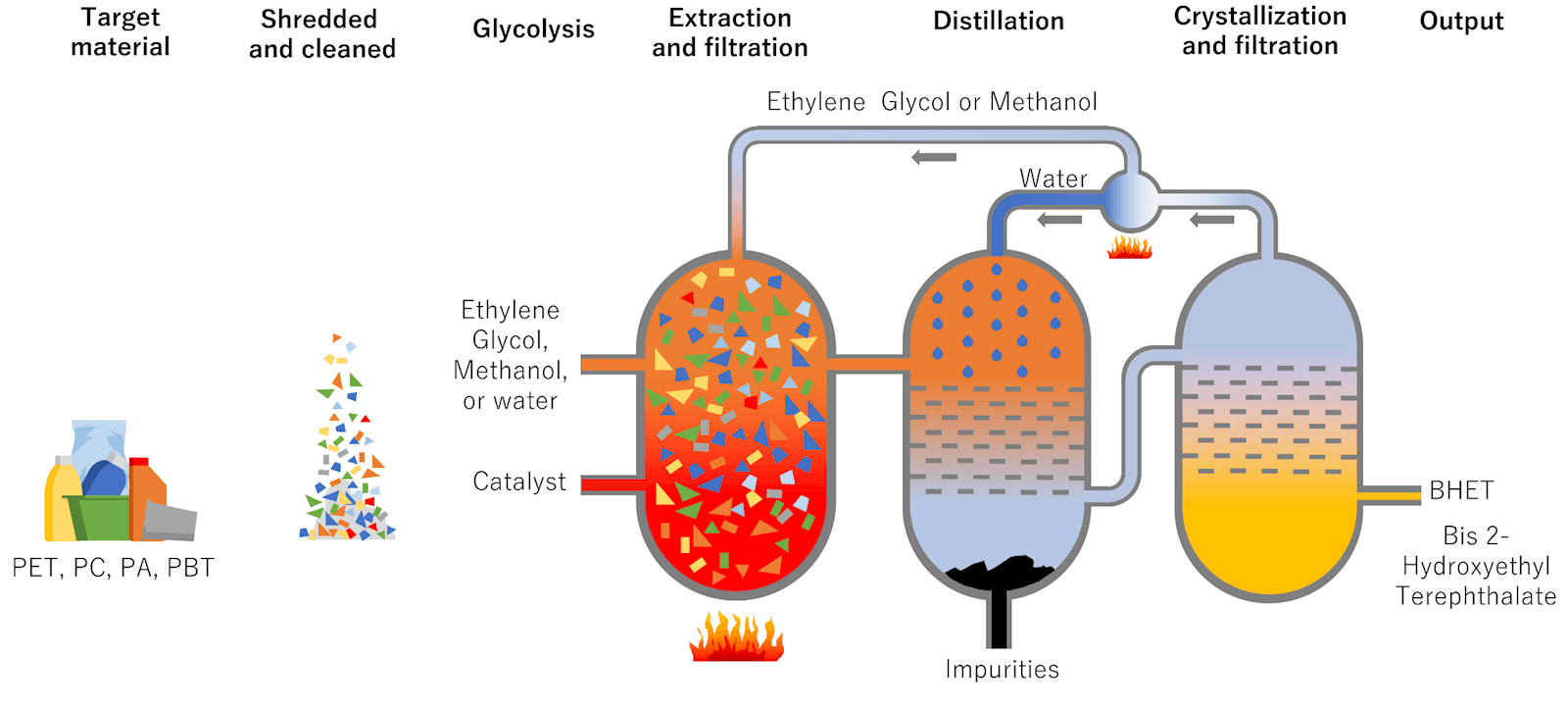
Solvolysis is a solvent-based process. The current main focus of solvolysis-based plastic recycling is PET, which comprises today’s water and soda bottles as well as many disposables and clear plastic packaging. It’s also called glycolysis or methanolysis or hydrolysis. The typical process might lead to monomers used to make virgin PET.
The advantages of all these chemical recycling technologies are that the output can be used in food contact and other critical applications. The U.S. Food and Drug Administration, the European Food Safety Authority, and similar authorities around the globe have approved PET bottles to be recycled mechanically back into food contact applications, given certain requirements are met. For other resins and applications, like non-bottle applications, particularly films, the options for circularity into high-value and food contact applications is limited without some form of chemical recycling.
The biggest disadvantage of these chemical recycling technologies is cost versus mechanical recycling and the need for extra sorting to make sure you receive just what you want to have the process work properly.
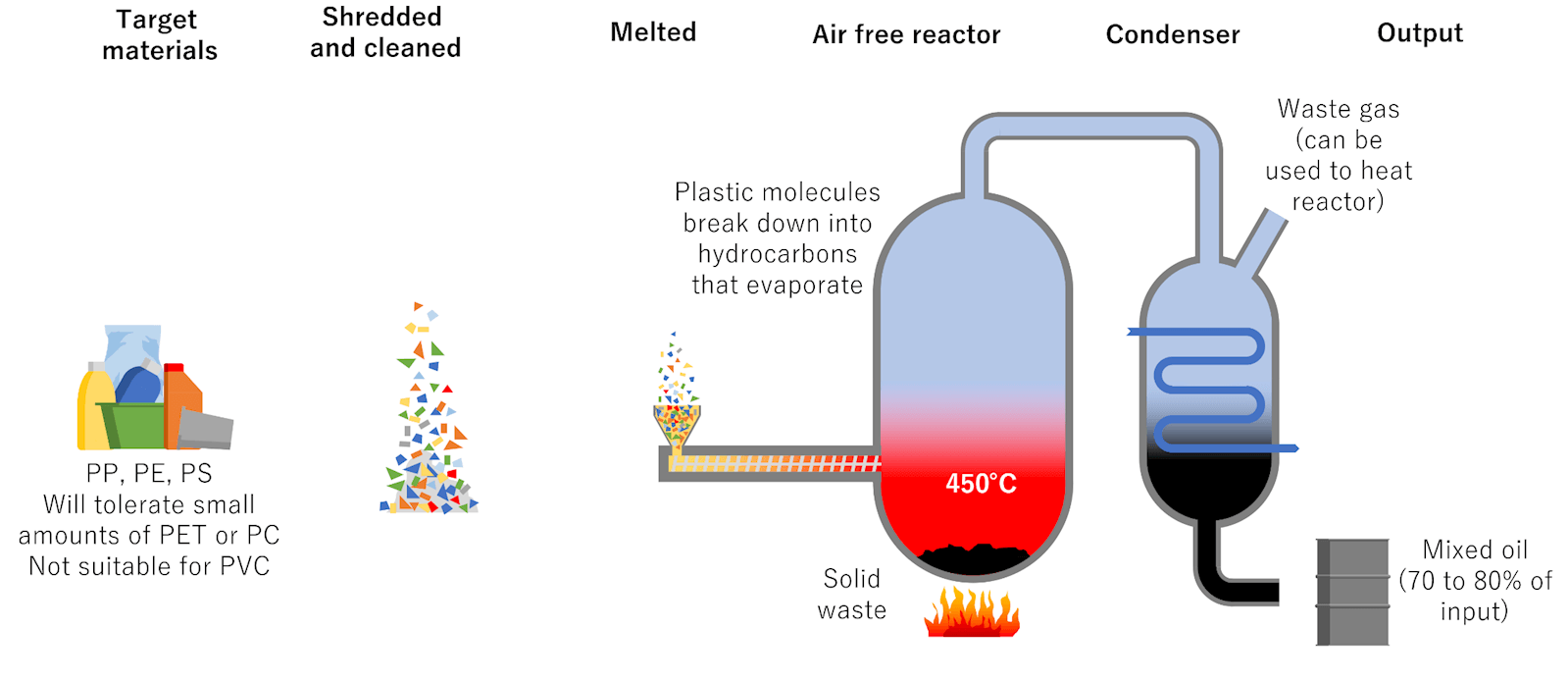
Pyrolysis: Sometimes called ‘plastics to fuel’, turns non-recycled plastics from municipal solid waste (garbage) into a synthetic crude oil that can be refined into diesel fuel, gasoline, heating oil or waxes. Using pyrolysis to convert non-recycled plastics into ultra-low sulfur diesel (ULSD) fuel reduces greenhouse gas emissions by 14% and water consumption by 58%, and it saves up to 96% in traditional energy use as opposed to ULSD from conventional crude oil. Many plastics include additives to give it specific property
Some polymers include additives during manufacture. Other polymers include additives during processing into their finished parts. Additives are incorporated into polymers to alter and improve basic mechanical, physical or chemical properties. Additives are also used to protect the polymer from the degrading effects of light, heat, or bacteria; to change such polymer processing properties such as melt flow; to provide product colour; and to provide special characteristics such as improved surface appearance, reduced friction, and flame retardancy.
Pyrolysis can remove these harmful chemicals and additives so any plastics made from this method will be additive-free. Mechanical recycling is not able to remove harmful and toxic additives so they are automatically recycled along with the plastic product.
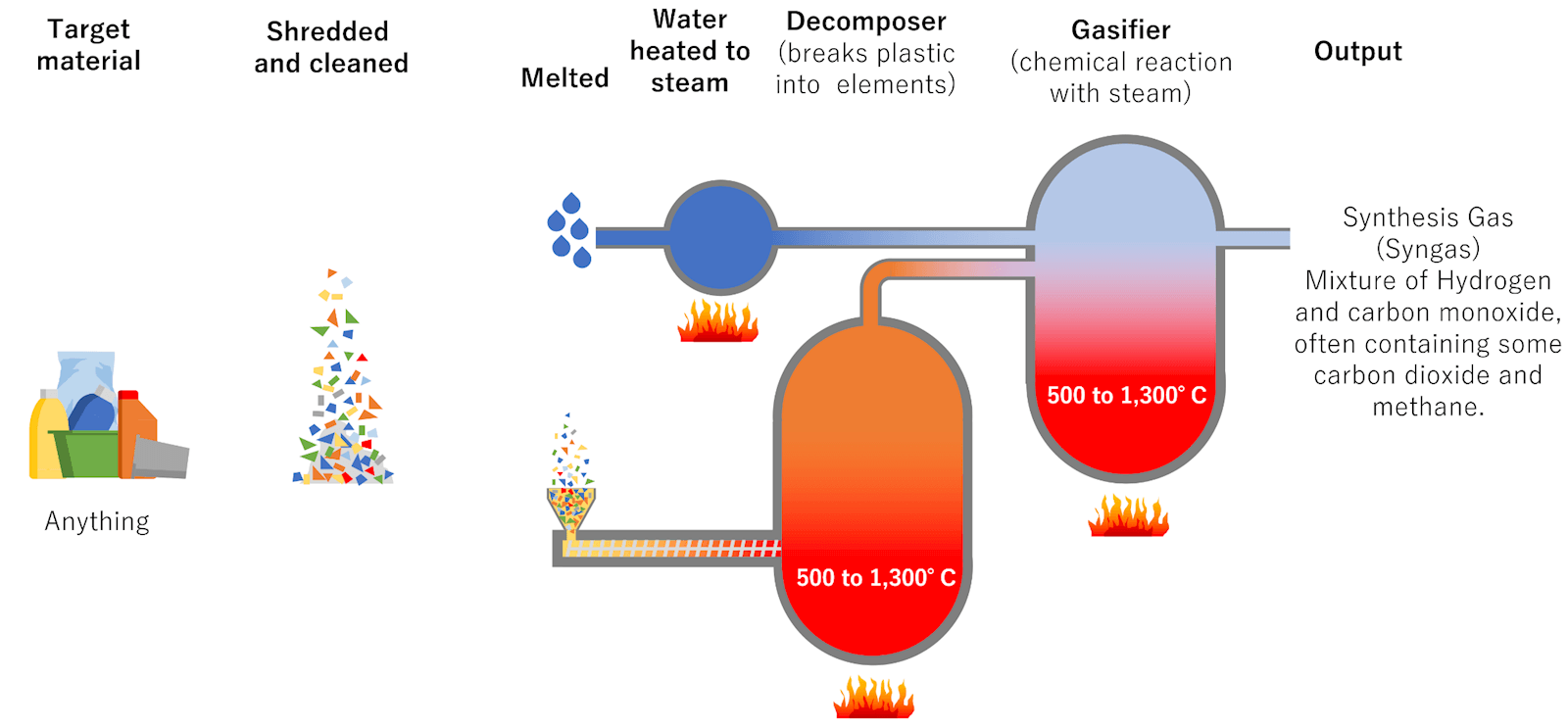
Gasification: By partial oxidation with a gasification agent, gasification refers to the chemical and thermal conversion of carbon-based materials into a primarily gaseous output (usually air, oxygen, or steam). Temperature ranges from 800 to 1,100°C when using air as an oxidant, and up to 1,500°C when using oxygen. This partial oxidation produces a ‘Syngas’ (a mixture of hydrogen, carbon monoxide and some carbon dioxide). The ‘Syngas’ can produce a variety of chemicals (methanol, ammonia, hydrocarbons, acetic acid) for plastics production as well as fuel and fertiliser.
While most gasification processes are exothermal, that is, they generate heat, some of the associated reactions are endothermal and require heat, which could be provided by steam as the gasification agent. Gasification of mixed waste has been used for some time.
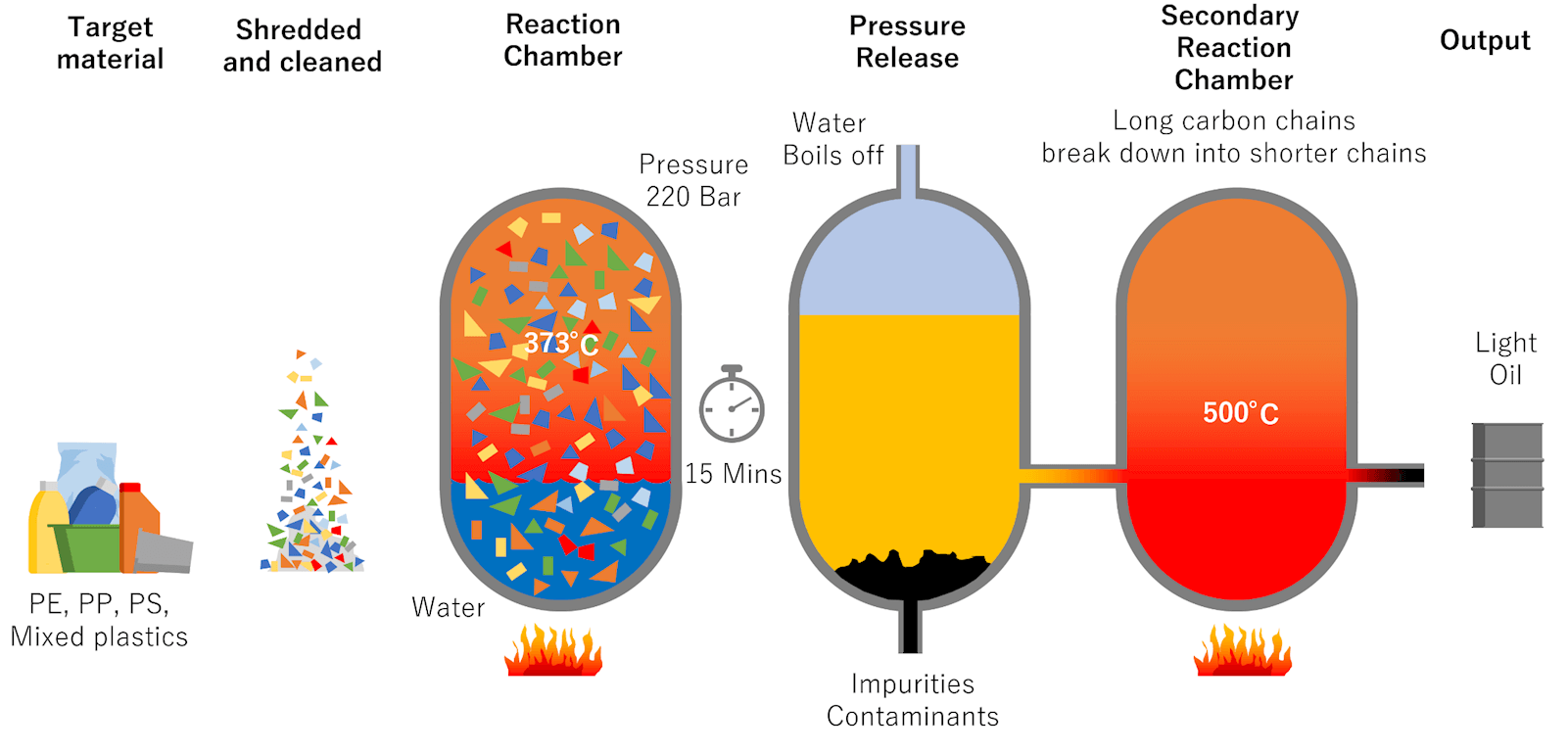
Hydrothermal Treatment: The process applies heat and pressure to waste plastic to generate a plastic melt stream which is then mixed with steam and heated to supercritical conditions (> 373 °C, > 220 bar). Polymers are broken down (cracked) into shorter-chain hydrocarbons in this environment. The reaction mixture is then passed through a reactor system and depressurised, with products then fractionated based on boiling points. The process is flexible and produces a range of liquid and gaseous hydrocarbons which can be used as feedstocks to make a range of products, such as new plastics and other chemicals.
Visit the Knowledge Centre
For more information on chemical recycling, recyclable resources and reprocessing visit the Knowledge Centre to access reports, research, videos, infographics, articles and images covering 50+ different topics.
Basic terms
Depolymerization: (or depolymerisation) is the process of converting a polymer into a monomer or a mixture of monomers.
Olefins: Olefin is a compound comprising hydrogen and carbon, with at least one pair of carbon atoms. Both petrol and diesel contain several different hydrocarbon molecules. Paraffins, olefins and aromatics account form most hydrocarbons in petrol, while diesel is mostly paraffins, aromatics and naphthenes.
Dissolution: Polymers are dissolved in a selected solvent so the polymer can be separated from any contamination before being precipitated back out and re-used as a polymer. Dissolution does not affect the chemical composition of the polymer.
References:
- Molecules images: Freepik from Flaticon
- Wikipedia: What is plastic?
- Oxford English Dictionary: Monomer
- American Chemistry Council: How plastics are made
- Americal Chemistry Council: What is chemical recycling?
- Chemical & Engineering News: Plastic has a problem; is chemical recycling the solution?
- Image: All chemical recycling images from the British Plastic Federation ‘Overview of Key Processes‘
- ‘Chemical Recycling Types: Advantages and Disadvantages‘ by Tony Kingsbury, published in GLG Insights, 17 May 2022.
References:
- ‘A Review on Gasification and Pyrolysis of Waste Plastics‘ by Hamad Hussain Shah, Muhammad Amin, Amjad Iqbal, Irfan Nadeem, Mitjan Kalin, Arsalan Muhammad Soomar and Ahmed M. Galal. Published in Frontiers of Chemicals, 03 February 2023
- Plastic Recyclers Europe: Chemical recycling
- Blue Vision Intelligence: Mechanical, energy or chemical. How different types of recycling work
- Plastics Industry Association: Recycling 101: Advanced recycling
- ‘Recycling of plastic waste into fuels, a review’ by Hayelom Dargo Beyene, International Journal of Science, Technology and Society, 08 December 2014.
- Petro Industry News: An introduction to olefins